Acellular dermal matrices (ADM) were first used in 1994 to treat burns, and their usage has become more extensive including as a tool in breast reconstructions1,2. ADM is an acellular connective tissue graft that provides a scaffold for the patient’s own cells to grow3. Breast reconstruction supported with the use of ADM has been shown to improve aesthetic outcome and reduce complications4,5. ADM can support many aspects of breast reconstruction including: the expansion of the submuscular pocket, symmastia (convergence of breasts at the midline, or missing cleavage), correcting inframammary fold irregularities, and others6. When choosing to undergo breast reconstruction there are many options to consider; one consideration is whether or not an ADM supported reconstruction will be the best choice. How does ADM work? Is it like an implant? What are the risks? Understanding this product and its utility in breast reconstruction can help patients make their decision with their plastic surgeon.
ADM is made up of collagen and extracellular matrix components; constituents that are naturally found in the body and won’t be detected as foreign by theimmune system6. ADM is acellular, meaning that all epitopes, an antigen recognizes by the immune system, that are associated with the identity of a cell are removed6. Extracellular matrix components are similar between species, and is preserved in ADM6. When ADM is implanted there are three possible responses from the immune system:integration, resorption, and encapsulation. When the ADM is not recognized by the immune system, it becomes integrated into the surrounding tissue by a vascularization of the tissue. ADM can also be recognized by the immune system and an inflammatory response results which leads to it’s breakdown, resorption6,7. Finally, encapsulation can occur which is a physiological reaction involvingscar tissue surrounding ADM6,8. In a study by Wong and colleagues at the Sloan-Kettering Cancer Center, it was found that the host response to ADM is similar to that of normal wound healing9.
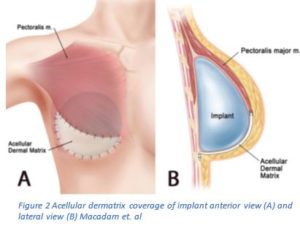
Traditionally, two-stage expander/implant reconstruction involved placing the expander beneath the muscle3,6. This was shown to lead to a number of issues including: restriction and tightness under the muscle and poor position of the implant, which may lead to reoperations3,6. Alternatively, the muscle is cut and secured to the ADM maintaining the shape of the breast and improving symmetry3,6. This method, as Dr. John Kim wrote, gives the surgeon increased control over the placement of the tissue expander, which will lead to improved aesthetic outcomes; it also creates a larger space for the implant, which will speed up the expansion process and finish the reconstruction quicker3.
It has been shown that ADM not only allows for the reduced frequency of office visits to complete expansion, but also reduces the risk of some complications. The application of ADM will reduce the risk of capsular contracture and implant malposition10,11. Although ADM is also associated with an increased risk of infection, seroma, and mastectomy flap necrosis10,11. Breast reconstruction with and without ADM has shown no differences in the risk of implant loss, reoperation, and total complications10,11. In a study from Harvard University, Ibrahim and colleagues found that mean aesthetic scores were higher in breast reconstructions supported with ADM5,12.
When working with a plastic surgeon to determine the best way to complete breast reconstruction, consider the use of ADM to help improve the aesthetic outcome.
1. Baxter RA. Intracapsular allogenic dermal grafts for breast implant-related problems. Plast Reconstr Surg 2003;112:1692–6.
2. Spear SL, Parikh PM, Reisin E, Menon NG. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008 May. 32(3): 418-425.
3. Kim, John, MD, and Neil Fine, MD. “Breast Reconstruction With Acellular Dermis.” : Overview, Periprocedural Care, Technique. Medscape, 4 Aug. 2015. Web. 23 Sept. 2016.
4. Lee, K. T., & Mun, G. H. (2016). Updated evidence of Acellular dermal matrix use for implant-based breast reconstruction: a meta-analysis. Annals of surgical oncology, 23(2), 600-610.
5. Macadam, S. A., & Lennox, P. A. (2012). Acellular dermal matrices: Use in reconstructive and aesthetic breast surgery. Canadian Journal of Plastic Surgery, 20(2).
6. Xu H, Wan H, Sandor M, et al. Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng Part A. 2008;14:2009-19.
7. Sandor M, Xu H, Connor J, et al. Host response to implanted porcine derived biologic materials in a primate model of abdominal wall repair. Tissue Eng 2008: Part A 14:2021.
8. Wong, A. K., Schonmeyer, B. H., Singh, P., Carlson, D. L., Li, S., & Mehrara, B. J. (2008). Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plastic and reconstructive surgery, 121(4), 1144-1152.
9. Lee, K. T., & Mun, G. H. (2016). Updated evidence of Acellular dermal matrix use for implant-based breast reconstruction: a meta-analysis. Annals of surgical oncology, 23(2), 600-610.
10. Zhao, X., Wu, X., Dong, J., Liu, Y., Zheng, L., & Zhang, L. (2015). A meta-analysis of postoperative complications of tissue expander/implant breast reconstruction using acellular dermal matrix. Aesthetic plastic surgery, 39(6), 892-901.
11. Ibrahim, A. M., Koolen, P. G., Ganor, O., Markarian, M. K., Tobias, A. M., Lee, B. T., … & Mureau, M. A. (2015). Does acellular dermal matrix really improve aesthetic outcome in tissue expander/implant-based breast reconstruction?. Aesthetic plastic surgery, 39(3), 359-368.
12. Nguyen, T. J., Carey, J. N., & Wong, A. K. (2011). Use of human acellular dermal matrix in implant-based breast reconstruction: evaluating the evidence. Journal of Plastic, Reconstructive & Aesthetic Surgery, 64(12), 1553-1561.
13. Vinsel, Anne. “Immediate Breast Reconstruction with Tissue Expander: First Surgery” Accessed 1 December 2016. http://img.medscape.com/pi/features/slideshow-slide/breast-reconstruction/fig11.jpg
