After having breast augmentation surgery with Dr. John Kim there will be a number of tips he will suggest to recover from surgery.
In particular, Dr. John Kim will recommend no heavy lifting or exercise for four to six weeks after surgery. The reason for this is to reduce the risk of the implant shifting as well as minimizing the risk of subclinical bleeding that may increase the patient’s risk of capsular contracture. Capsular contracture is designated one of the top reasons for reoperation [Wan]. So what is the risk of capsular contracture? According to reports written by three major implant companies Allergan, Mentor, and Sientra there is between a 2 to 15% risk of capsular contracture after primary breast augmentation (3-7 year follow up) [Caplin, Spear, Stevens]. At Northwestern Medical Group, Dr. John Kim will help advise you about how to reduce your risk of capsular contracture.
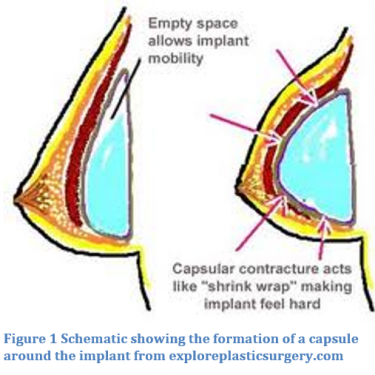
Capsular contracture is a condition where the natural scar tissue that forms around an implant becomes thick and contacts, which can cause aesthetic changes, hardening, or pain [Grigg]. When an implant is placed the body undergoes an immune response. The body’s natural response to the placement of a foreign body, like a breast implant, involves an immune response that recruits fibroblasts to make a fibrous capsule separating the implant from the body [Grigg].
There is a scale that measures the severity of contracture called the Baker Classification Scale: Class I the augmented breast is as malleable as the non-augmented, Class II augmented breast is less supple than the non-augmented, Class II the augmented breast is firmer and can be seen below the skin, and Class IV the augmented breast is firm, hard, painful, cold, and visibly distorted. There is still a lot to be learned about the mechanism by which the capsular hardens and why this thickening happens.
A number of papers have been published studying the incidence of capsular contracture and ways to avoid this complication. Capsular contracture is seen less when textured implants are used versus round, but this is attributed to the texture surrounding the implant not the shape [Wan]. The textured surface of the implant may disrupt the contractile forces around the implant, but at this time more studies are needed to explain this phenomenon [Headon]. Capsular Contracture is also less likely in submuscular implant placement verses prepectoral [Wan]. Although, Headon and colleagues wrote that implant placement in either plane can cause capsular contracture it is only that patients with submuscular placement have a lower risk.
The treatment for severe capsular contracture is re-operation; the fibrous capsule is removed and an implant replaced [Griggs]. In a review article by Wan and colleagues they considered what treatment option for capsular contracture would offer the best outcomes. The current gold standard for treating capsular contracture is “capsulectomy, site change, and implant exchange” [Wan]. In their literature review they found that whether a capsulectomy (partial or total) or capsulotomy is performed is based on the individual patient. Capsulectomy is a procedure where part or the entire fibrous capsule surrounding the implant is removed or manipulated. Capsulotomy is a procedure where the capsule is left in place and the implant is replaced. They found significant evidence that site change and implant exchange improve patient outcomes and reduce the chance of another case of capsular contracture.
If you notice any changes to your breasts after surgery make an appointment to see Dr. John Kim at Northwestern Medical Group.
1) Caplin, D. A. (2014). Indications for the use of MemoryShape breast implants in aesthetic and reconstructive breast surgery: long-term clinical outcomes of shaped versus round silicone breast implants. Plastic and reconstructive surgery, 134(3S), 27S-37S.
2) Headon, H., Kasem, A., & Mokbel, K. (2015). Capsular contracture after breast augmentation: an update for clinical practice. Archives of plastic surgery, 42(5), 532-543.
3) Institute of Medicine (US); Grigg M, Bondurant S, Ernster VL, et al., editors. Information for Women About the Safety of Silicone Breast Implants. Washington (DC): National Academies Press (US); 2000. A Report of a Study by the Institute of Medicine. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44775/
4) Spear, S. L., & Murphy, D. K. (2014). Natrelle round silicone breast implants: core study results at 10 years. Plastic and reconstructive surgery, 133(6), 1354.
5) Stevens, W. G., Harrington, J., Alizadeh, K., Broadway, D., Zeidler, K., & Godinez, T. B. (2015). Eight-year follow-up data from the US clinical trial for Sientra’s FDA-approved round and shaped implants with high-strength cohesive silicone gel. Aesthetic Surgery Journal, 35(suppl 1), S3-S10.
6) Wan, D., & Rohrich, R. J. (2016). Revisiting the management of capsular contracture in breast augmentation: a systematic review. Plastic and reconstructive surgery, 137(3), 826-841.
7) “Capsular Contracture Rates in Breast Augmentation.” Explore Plastic Surgery – Dr. Barry Eppley. N.p., 18 Sept. 2013. Web. 02 Apr. 2017.