The term autologous means derived from the same individual or involving a patient as both the donor and the recipient1. There are numerous ways that humans utilize their own tissues such as blood transfusion, transplanting one’s cells elsewhere in the body, and modeling the body’s tissues like clay to change appearance; for example in breast reconstruction. There are many tissues one can use to mold a new breast including the abdomen, back, buttock, and thighs. Each of these types of autologous procedures have their benefits, and over time have transformed to create the safest procedure for patients. The abdomen was an obvious site for harvesting tissue to craft a new breast. The abundance of soft tissue in the abdominal area allows for enough volume in the reconstructed breast that an additional implant is not needed2. Since the early 80’s techniques capitalizing on the tissue make-up of the abdomen have inspired the transverse rectus abdominus myocutaneous (TRAM) flap which later developed into the deep inferior epigastric perforator (DIEP) flap2-5.
The first use of the TRAM flap was published in 1982 by Hartrampf6. Then in 1988 they published a 7-year detailed description of technique, patient selection, and the incidence of complications as well as means to prevent them7. The procedure was found to be effective and safe. Since its inception in the 1980s the TRAM flap has developed to minimize risk and has inspired a number of other autologous techniques.
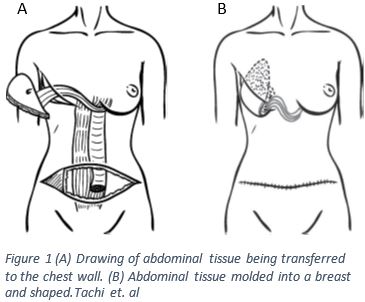
The TRAM flap involves removing a skin island on the abdomen; the skin island also involves the adipose tissue, blood supply, and a small amount of rectus muscle as seen in figure 13. The skin island for this type of flap is taken between the umbilicus (belly button) and the pubic region4. The rectus abdominus inserts at the most inferior point of the sternum, the xiphoid process, along with attachments along the ribs and originates at the pubic symphysis where the two pubic bones of the pelvis meet1,2. Once the flap is created it is tunneled superiorly into the mastectomy pocket as seen in figure 1 A3,4. It is critical that a new blood supply is generated otherwise the skin flap will have no access to nutrients and could become necrotic requiring more surgery.
The rectus abdominal muscle is supplied by three blood supplies the lower intercostal arteries and the deep superior and inferior epigastric vessels (SIEV and DIEV)1. According to Mathes and Nahai it is classified as a type III muscle8. This classification is determined by the way blood circulates through the muscle. The superior epigastric artery supplies blood to the upper portion of the rectus abdominus1,8. The inferior epigastric artery runs travels along the posterior surface of the rectus abdominus and supplies the lower portion of the muscle1,8. The superior and inferior epigastric arteries form an anastomoses, a connection of vessels, at the level of the umbilicus1,8. Due to the circulation pattern of this type of muscle, it may be split, and blood can still supply both sections8.
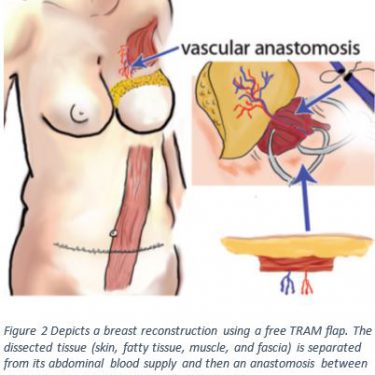
Techniques that harvest tissue flaps and preserve the muscle reduce the occurrence of donor site morbidity5,9,10. Although, this technique is more complex and takes longer to recover from, it may prevent abdominal wall complications after a conventional TRAM flap5,11. Figure 2 shows a drawing of this muscle sparing technique called the free TRAM flap. The free TRAM flap comes with its own risks due to the longer surgical time; such as a risk of flap necrosis due to venous thrombosis, a clot within the veins4.
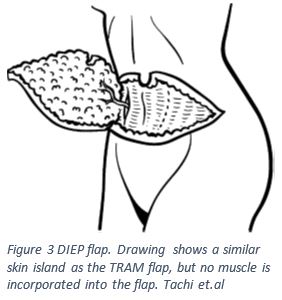
In 1989 Koshima and Soeda published their findings that a large flap without muscle can survive with an attached blood supply, in this case using the deep inferior epigastric vessles (DIEV)12,13. Similar to the free TRAM flap, the deep inferior epigastric perforator (DIEP) flap involves removing a tissue island from the lower abdomen5,12,13. In order to create this tissue flap the surgeon will select large vessels that branch from the inferior epigastic artery5,12,13. The surgeon will make an incision to the level of the abdominal fascia, and at this point look for branches of the inferior epigastric artery that may be viable; once the vessel of choice has been found it will be clipped and reconnected to a blood supply in the breast capsule as seen in Figure 35,12,13. This technique is significantly less disruptive to the rectus abdominus and reduces the incidence of donor site morbidity and patient recovery time5,14. In a 2016 study comparing rectus abdominus muscle function in patients who underwent DIEP versus a free TRAM found that the patients who with the DIEP flap had significantly better muscle function15.
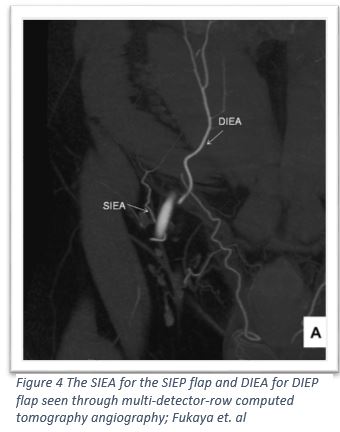
At the end of the 20th century a new autologous abdominal flap was created, taking advantage of the subdermal blood vessels16. Similar to the above procedures the SIEA flap involves creating a skin island in the lower abdomen from around the umbilicus to the pubic bone; the difference is that the incision doesn’t go deeper than the subcutaneous layer17,18. The advantage of the SIEP flap is that it does not involve disrupting the rectus abdominus and the vasculature of the flap is a smaller interwoven network instead of a large vessel supplying large muscular zones as seen in the DIEP flap16. In addition the dissection of the skin island is only subcutaneous without disrupting the abdominal fascia, which allows this procedure to claim the lowest risk for donor site morbidity17,19. Not all patients have the SIE vessels necessary, around 65% of the population is eligible for this procedure17,20. In a 2016 study of 145 flaps, researchers found that there was a 4.8% incidence of flap loss, 4.8% incidence of complete flap loss, and 1.4% incidence of mastectomy flap necrosis17.
Since its debut over twenty years ago DIEP has been the most popular autologous procedure21. With advances in technology and improvements in technique DIEP and SIEA flap reconstruction has provided an answer to the question of how to utilize the tissue available in the abdomen to create a new breast. Further innovations such as these will lead to further progress and means to improve patient outcomes.
1. Gray, Henry. Anatomy of the Human Body. Philadelphia: Lea & Febiger, 1918; Bartleby.com, 2000. bartleby.com/107/. Accessed 11/17/2016
2. Caterson, Stephanie A., Matthew J. Carty, Lydia A. Helliwell, Charles A. Hergrueter, Julian J. Pribaz, and Indranil Sinha. “Evolving options for breast reconstruction.” Current problems in surgery 52, no. 5 (2015): 192-224.
3.Tachi, M., & Yamada, A. (2005). Choice of flaps for breast reconstruction. International journal of clinical oncology, 10(5), 289-297
4.Teymouri, H. R., Stergioula, S., Eder, M., Kovacs, L., Biemer, E., & Papadopulos, N. A. (2006). Breast reconstruction with autologous tissue following mastectomy. Hippokratia, 10(4), 153.
5. Pinel-Giroux, F. M., El Khoury, M. M., Trop, I., Bernier, C., David, J., & Lalonde, L. (2013). Breast reconstruction: review of surgical methods and spectrum of imaging findings. Radiographics, 33(2), 435-453
6. Hartrampf C, Scheflan M, Black P (1982) Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg 69:216–225
7. Hartrampf Jr, C. R. (1988). The transverse abdominal island flap for breast reconstruction. A 7-year experience. Clinics in plastic surgery, 15(4), 703.
8. Mathes, S. J., & Nahai, F. (1981). Classification of the vascular anatomy of muscles: experimental and clinical correlation. Plastic and reconstructive surgery, 67(2), 177-187.
9. Kroll SS, Schusterman MA, Reece GP, Miller MJ, Robb G, Evans G. Abdominal wall strength, bulging, and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg 1995;96(3):616–619.
10. Andrades P, Fix RJ, Danilla S, et al. Ischemic complications in pedicle, free, and muscle sparing transverse rectus abdominis myocutaneous flaps for breast reconstruction. Ann Plast Surg 2008;60(5): 562–567.
11. Schusterman MA, Kroll SS, Weldon ME. Immediate breast reconstruction: why the free TRAM over the conventional TRAM flap? Plast Reconstr Surg 1992;90(2):255–261; discussion 262.
12. Koshima, I., & Soeda, S. (1989). Inferior epigastric artery skin flaps without rectus abdominis muscle. British journal of plastic surgery, 42(6), 645-648.
13. Allen RJ, Treece P. Deep inferior epigastric perforator flap for breast reconstruction. Ann Plast Surg 1994;32(1):32–38.or plastic surgery
14. Garvey PB, Buchel EW, Pockaj BA, et al. DIEP and pedicled TRAM flaps: a comparison of outcomes. Plast Reconstr Surg 2006;117(6):1711–1719; discussion 1720–1721.
15. Seidenstuecker, K., Legler, U., Munder, B., Andree, C., Mahajan, A., & Witzel, C. (2016). Myosonographic study of abdominal wall dynamics to assess donor site morbidity after microsurgical breast reconstruction with a DIEP or an ms-2 TRAM flap. Journal of Plastic, Reconstructive & Aesthetic Surgery, 69(5), 598-603.
16. Holm, C., Mayr, M., Höfter, E., & Ninkovic, M. (2007). The versatility of the SIEA flap: A clinical assessment of the vascular territory of the superficial epigastric inferior artery. Journal of plastic, reconstructive & aesthetic surgery, 60(8), 946-951.
17. Park, J. E., Shenaq, D. S., Silva, A. K., Mhlaba, J. M., & Song, D. H. (2016). Breast Reconstruction with SIEA Flaps: A Single-Institution Experience with 145 Free Flaps. Plastic and reconstructive surgery, 137(6), 1682-1689.
18. Arnez, Z. M., Khan, U., Pogorelec, D., & Planinsek, F. (1999). Breast reconstruction using the free superficial inferior epigastric artery (SIEA) flap. British journal of plastic surgery, 52(4), 276-279.
19. Selber, J. C., Samra, F., Bristol, M., Sonnad, S. S., Vega, S., Wu, L., & Serletti, J. M. (2008). A head-to-head comparison between the muscle-sparing free TRAM and the SIEA flaps: is the rate of flap loss worth the gain in abdominal wall function?. Plastic and reconstructive surgery, 122(2), 348-355.
20. Taylor GI, Daniel RK. The anatomy of several free flap donor sites. Plast Reconstr Surg. 1975;56:243–253.
21. Healy, C., & Allen, R. J. (2014). The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. Journal of reconstructive microsurgery, 30(02), 121-126.
22. Fukaya, E., Kuwatsuru, R., Iimura, H., Ihara, K., & Sakurai, H. (2011). Imaging of the superficial inferior epigastric vascular anatomy and preoperative planning for the SIEA flap using MDCTA. Journal of Plastic, Reconstructive & Aesthetic Surgery, 64(1), 63-68.